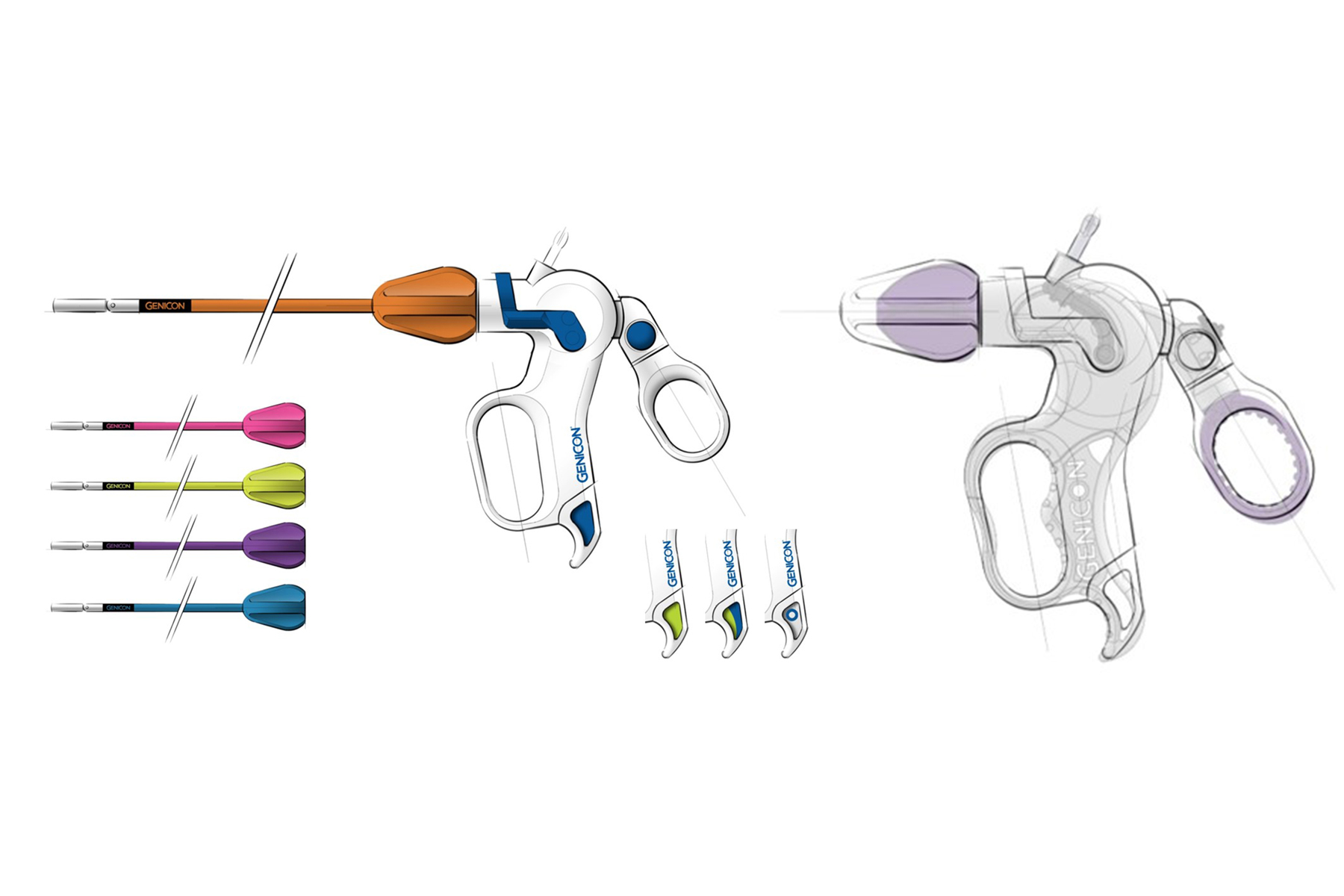
Discovery | Design | Development
For nearly three decades, BlackHägen Design has provided an integrated approach to research and development to industry, focusing on mission and safety-critical devices, including Class I, II, and III medical devices. We provide R&D support to entrepreneurs and start-up entities through to Fortune 50, multi-national and government-sponsored corporations.
Services may be specific such as Conceptual Design or Usability Testing, or turn-key from product definition through to pilot-production and validation. Our internal Quality System is designed to support regulated industries such as medical device development, conforming to FDA’s Quality System Regulation (QSR) and ISO 13485. We are periodically audited by Fortune 50 medical device manufacturers to ensure adequate compliance to the client’s QMS and are on the Approved Supplier List for many of the top 20 corporations.